METHYLENE BLUE TEST (MBT)
Methylene blue is a synthetic organic compound with the chemical formula C₁₆H₁₈N₃SCl. It is a cationic dye, meaning that it carries a positive charge and is attracted to negatively charged surfaces. It is soluble in water and alcohol.
A rapid estimate of the amount of montmorillonite present in a mud or clay can be obtained by means of the methylene blue test. This test measures the amount of methylene blue dye adsorbed by clays, which is a function of their base exchange capacity. Since montmorillonite has a much larger base exchange capacity than other clay minerals, the test has come to be regarded as measure of the amount of montmorillonite present, reported as estimated bentonite content.
Reagents
- Methylene blue solution:1 ml = 0.01 milliequivalents.
- (This solution has a concentration where 1 milliliter (ml) equals 0.01 illiequivalents. It’s prepared by dissolving 3.74 grams of USP-grade methylene blue (C₁₆H₁₈N₃SCl•3H₂O) per liter)
- Hydrogen peroxide, 3% solution.
- 5 N sulfuric acid solution.
Procedure
Sample Preparation
- Measure 2 ml of drilling mud (or a volume that requires 2–10 ml of methylene blue for titration).
- Add the mud to 10 ml of water in a 150-ml Erlenmeyer flask.
- Add 15 ml of 3% hydrogen peroxide and 0.5 ml of 5 N sulfuric acid.
- Swirl the contents thoroughly and then gently boil the mixture for 10 minutes.
- After boiling, dilute the mixture to about 50 ml with water.
Note: Hydrogen peroxide treatment is used to remove interference from organic substances such as CMC, polyacrylates, lignosulfonates, and lignites, which can also absorb methylene blue.
Titration
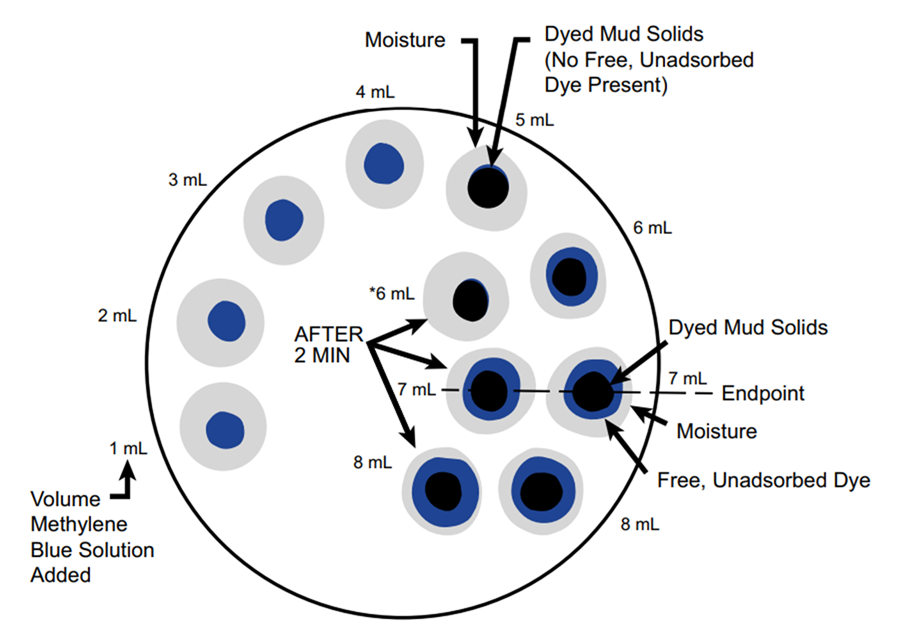
- Add methylene blue solution in 0.5 ml increments from a burette or pipette.
- After each addition, stopper the flask and shake vigorously for about 30 seconds.
- While the solids are still suspended, withdraw a drop with a glass rod and place it on filter paper.
- Look for a greenish-blue ring around the stained solids — this indicates that the clay can no longer absorb more dye.
Endpoint Confirmation
- Once a greenish-blue halo is observed, shake the flask for an additional 2 minutes and test another drop.
- If the ring is still present, the endpoint has been confirmed.
- If not, continue adding methylene blue in 0.5 ml increments, shaking and testing after each addition, until the ring consistently appears.
- Record the ml of methylene blue solution used.
Calculations
METHYLENE BLUE CAPACITY OF SHALE
Sample Preparation
- Accurately weigh approximately 1 gram of dry, ground shale.
- Transfer to a 150-ml Erlenmeyer flask
- Add 50 ml of deionized water and 0.5 ml of 5 N sulfuric acid.
- Gently boil the slurry for 10 minutes.
- Allow the sample to cool before titration.
Titration
- Add 0.01 N methylene blue solution in 0.5 ml increments.
- Follow the same endpoint detection procedure as above using filter paper.
Calculations
MBC (lb/bbl) = CEC x 5
MBC (kg/m3) = CECx14
The above formula used to calculate the Cation Exchange Capacity (CEC) of shale is based on the assumption that the methylene blue (MB) solution has a known concentration — typically:
1 mL of MB = 0.01 milliequivalents (meq)
This value comes from the normality (or strength) of the MB solution prepared in the lab. The full formula is:
This formula is only valid if the methylene blue solution you’re using contains 0.01 meq of dye per ml..
What If the Methylene Blue Solution Has a Different Concentration?
So, for example, If you’re using 0.005 meq/ml MB solution, the formula becomes:
Example to Illustrate
Suppose you used 3 ml of methylene blue for a sample that weighs 1 gram:
This tells you that 1—grams of this shale would have the capacity to hold 3 milliequivalents of positively charged ion (cations).
This means the shale contains reactive clay minerals (such as smectite or montmorillonite) with negatively charged surfaces that can adsorb 3 milliequivalents of cations per 100 grams of rock. A higher CEC value generally indicates higher clay activity, swelling potential, and greater impact on mud properties — such as viscosity, stability, and fluid loss control. This is critical information when evaluating how shales will interact with water-based drilling fluids.
All the content—whether it’s drilling fluids insights or coding tutorials—is freely available to support fellow engineers and developers. If you find the articles helpful and would like to support this effort, please consider making a voluntary donation via e-transfer to syeds@hotmail.com with security code 129. Thank you! 🙏